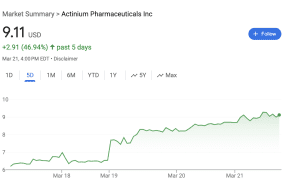
TC Biopharm (NASDAQ: TCBP) (NASDAQ: TCBPW) today announced the dosing of its first three patients within its Phase 2b clinical trial of OmnImmune®, an allogeneic unmodified cell therapy focused on treating Acute Myeloid Leukemia (AML).
TC Biopharm (NASDAQ: TCBP) is a clinical stage biotechnology company developing platform allogeneic gamma-delta T cell therapies for cancer.
As per the press release, the initial 5 patients in the trial are deemed a “safety cohort”, spaced two weeks apart with safety review by an oversight board to confirm no drug related toxicity issues, subsequent to 5 patients being dosed the study will advance to open enrollment. This safety cohort is in line with TCBP’s step-wise clinical trial advancement, moving from donor matching in the Phase 1b to a universal donor model with no HLA matching of donor to patient.
The launch of our Phase 2B trial is a key milestone in the development of our lead therapeutic, OmnImmune®, for patients with AML and for TC BioPharm’s emerging pipeline of ‘off-the-shelf’ gamma-delta T cell therapies,” said Bryan Kobel, CEO of TC BioPharm. “This study design includes a 5 patient safety cohort prior to open enrollment, we expect to complete the safety cohort before the end of 2022. The next step in the study is a 19 patient interim review, which will allow TCBP to review dosing and increase dosing to a higher level should our team deem it necessary for efficacy, or we can elect to maintain our current dosing level of 7×10^7 or 700 million cells per dose. We look forward to moving ahead with our Phase 2b trial with a target for open enrollment in January 2023, as well as our efforts to expand our clinical efforts in the US in the first half of 2023.”
TC BioPharm’s Phase 2B trial, dubbed ACHIEVE, will enroll adults diagnosed with AML who have either relapsed or are refractory to prior treatments as well as a cohort for patients with myelodysplastic syndromes (MDS), conditions that can occur when the blood-forming cells in the bone marrow become abnormal. The trial is expected to enroll approximately 37 patients.
Recently, TCBP received approval from the UK regulatory body MHRA to apply an 18-month extrapolated shelf life to its current allogeneic cell therapy product, OmnImmune®. This award enables TC BioPharm to ship and store sufficient product which can then be held cryopreserved at clinical sites to fulfill the requirements not only for its current Advanced Myeloid Leukemia trial ACHIEVE, but also for future clinical trials and eventually commercial treatment of patients. Additionally, TC BioPharm’s in-house quality control department will continue to gather data in order to extend the shelf-life of OmnImmune® (1)
TC BioPharm Ltd (NASDAQ:TCBP)’s platform technology allows Company to design specific therapies to treat a range of cancers and infectious diseases with off-the-shelf allogeneic gamma-delta T cell (GDT) products. The Company’s pipeline includes OmnImmune, ImmuniStim and CAR-T programs. OmnImmune is an unmodified allogeneic gamma-delta T cell product, being used for the treatment of acute myeloid leukemia (AML). ImmuniStim is an unmodified allogeneic gamma-delta T cell product, being used for the treatment of COVID-19. The Company also provides in-house and partner programs at the pre-clinical stage focused on developing CAR-modified allogeneic gamma delta T-cell products targeting solid and hematological indications.
TCBP recently announced that the company has completed its scientific advisory board (SAB) to advance new therapeutic trials and establish strategic relationships within the cell therapy sector (2)
“I appreciate the esteemed members of the SAB joining myself and TCBP in our efforts in building this innovative cell therapy development company,” said Bryan Kobel, Chief Executive Officer. “Cell therapy offers a myriad of opportunities in which to expand our gamma delta platform, and I believe that the breadth of expertise and knowledge represented on our SAB puts us in a unique position to connect with multiple companies across various disciplines in this space.”
“Current advancements within cell therapy represent an exciting leap forward in advancing our ability to understand and treat various blood cancers,” said Dr. Mark Bonyhadi, Scientific Advisory Board member at TC BioPharm. “I look forward to working with these strategic additions to our team as we advance our clinical trials and expand our strategic relationships.”
What sets TCBP apart from its competitors?
TCBP claims itself to be the Leading Gamma-Delta T-Cell Company. Vertically Integrated Operations, along with building exceptional teams and capabilities allows TC BioPharm to move quickly, providing unique ability to rapidly respond to internal discovery and external factors. GDTs provide a unique product development platform, which will enable next generation solid-tumor CAR-T and infectious disease therapies. Visit https://ir.tcbiopharm.com/ to learn more about this small cap biotech company.
Watch this Video To Learn More About TCBP and its product pipeline
https://www.youtube.com/embed/uf-r6O9XRmg
Other companies in TC BioPharm (Holdings)’s space includes: Legend Biotech (NASDAQ:LEGN), Sarepta Therapeutics (NASDAQ:SRPT), Exact Sciences (NASDAQ:EXAS), Neurocrine Biosciences (NASDAQ:NBIX) and Karuna Therapeutics (NASDAQ:KRTX).
Stocks making the biggest moves premarket: Best Buy, Dick’s Sporting, Abercrombie & Fitch, MDT, ZM, DELL, and URBN. Some of the most active stocks yesterday on the street included Imago Biosciences Inc (NASDAQ:IMGO), ICU Medical Inc (NASDAQ:ICUI), Sotera Health Co (NASDAQ:SHC) and Meta Materials Inc (NASDAQ:MMAT). In addition, shares of Walt Disney Co (NYSE:DIS) rose more than 7% after the company announced that former CEO Bob Iger would return to the helm of the entertainment giant, replacing Bob Chapek immediately.
About InvestorBrandMedia.com – The team at InvestorBrandMedia has decades of strategy-driven, extensive media experience in helping navigate our clients through ever changing Digital Landscape. We specialize in a variety of marketing strategies that help promote our client visibility across multiple channels helping them expand the digital footprint in an ever-complex environment. InvestorBrandMedia.com specializes in ticker tagging services. Targeting the right audience is essential. Without effective distribution, a company’s message will fall on deaf ears or play to an empty room. Our growing distribution network consists of a diverse network of finance-focused blogs and websites, producing a robust mix of organic and cultivated interest. Let us help you jumpstart a rich following of qualified leads you can build on. Ticker Tagging is the process wherein your company is included with other sector related NASDAQ, NYSE, AMEX companies showcasing your news within a ticker tagged PR. The ticker tags are distributed to approximately 400 business and financial news portal including Benzinga, Yahoo, Google News, Digital journal and MarketWatch. Companies looking for IR/PR services can contact us directly at investorbrandmedia@gmail.com. This is a compensated post. Visit https://investorbrandmedia.com/disclaimer/ for more info.